Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
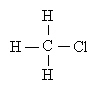 The name of the above compound is a. | 1-chloromethane | c. | methyl chlorine | b. | chloromethane | d. | none of the
above |
|
|
|
2.
|
Which of the following statements is not a property of an
aldehyde?
a. | it contains oxygen | c. | it is soluble in water | b. | it can form
esters | d. | none of the
above |
|
|
|
3.
|
A water molecule with an alkyl group replacing one of its hydrogen atoms is
called a(n)
a. | alcohol | c. | aldehyde | b. | ketone | d. | carboxylic acid |
|
|
|
4.
|
The IUPAC name for
CH3CH2CH2CH2CH(OH)CH3 is
a. | 2-hexanol | c. | 4-hexanol | b. | hexanol | d. | both b and c |
|
|
|
5.
|
The IUPAC name for
CH3CH2CH2OCH2CH3
a. | propyl ethane | c. | methyl propane | b. | ethyl propane | d. | none of the
above |
|
|
|
6.
|
What is the structural formula for 3-heptanol?
a. | 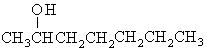 | c. | CH3CH2CH2-O-CH2CH2CH2CH3 | b. | 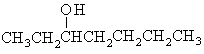 | d. | CH3CH2CH2CH2CH2CH2CH2OH |
|
|
|
7.
|
The IUPAC name for
CH3CH2CH2CH2CH2CH2CH2-O-CH2CH3
a. | nonane | c. | ethoxyheptane | b. | heptyl ethane | d. | 3-ethoxyheptane |
|
|
|
8.
|
Which of the following is not true of ethers?
a. | they are polar | c. | they can dissolve nonpolar substances | b. | they form hydrogen
bonds | d. | they are
flammable |
|
|
|
9.
|
To which group of organic compounds does cholesterol belong?
a. | alcohols | c. | ketones | b. | aldehydes | d. | carboxylic
acids |
|
|
|
10.
|
Which of the following groups does not contain carbonyl groups?
a. | alcohols | c. | ketones | b. | aldehydes | d. | carboxylic
acids |
|
|
|
11.
|
An amine is characterized by what functional group?
a. | -CO2CH3 | c. | -CO2H | b. | -NH2 | d. | -CHO |
|
|
|
12.
|
Which of the following is an alcohol?
|
|
|
13.
|
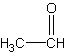 The compound above is classified as a(n) a. | alkane | c. | aldehyde | b. | carboxylic acid | d. | ketone |
|
|
|
14.
|
The formula for methyl ethanoate is which of the following?
|
|
|
15.
|
Which of following chlorine compounds has had a negative influence on
Earth's ozone layer?
a. | chlorofluorocarbons (CFCs) | b. | hexachlorobenzene | c. | dichlorodiphenyltrichloroethane (DDT) | d. | tetrachloromethane (carbon
‘tet’) |
|
|
|
16.
|
Esters are
a. | often fragrant | c. | found in some types of flavoring | b. | essential oils in
fruits and flowers | d. | all of
the above |
|
|
|
17.
|
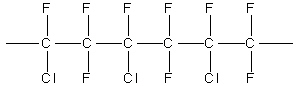 What is the monomer for the polymer Kel-F, shown
above?
|
|
|
18.
|
Why is a minor spill of organic solvents potentially dangerous?
a. | it flows easily | c. | it soaks into fabrics nearby | b. | it vaporizes
quickly | d. | all of the
above |
|
|
|
19.
|
Why is delivering organic solvents via spray cans especially hazardous?
a. | large amounts are delivered at once | c. | small droplets easily mix with
air | b. | the contents are under pressure | d. | none of the
above |
|
|
|
20.
|
Which of the following is a sensible precaution when working with organic
solvents?
a. | work in an enclosed space | c. | keep away from open
flames | b. | store the container below ground | d. | breathe deeply |
|