Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
A chemical model for CH4 would have
a. | a chain of 1 hydrogen and 4 carbons | b. | a chain of 4 hydrogens and 1
carbon | c. | 4 carbons with 4 hydrogens attached to them | d. | a single carbon with
4 hydrogens attached to it |
|
|
|
2.
|
The Bohr-Rutherford diagram shown of oxygen suggests that in ionic compounds,
oxygen would have a charge of 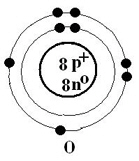
|
|
|
3.
|
The Bohr-Rutherford diagram shown of magnesium suggests that in ionic compounds,
magnesium would have a charge of 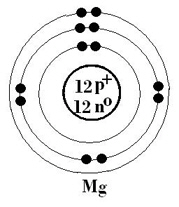
|
|
|
4.
|
In the chemical formula NaHCO3, there are
a. | four different elements | b. | four non-metallic elements | c. | three atoms in
total | d. | twelve atoms in total |
|
|
|
5.
|
The Bohr-Rutherford diagram shown represents a 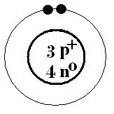 a. | neutral atom | b. | neutral molecule | c. | -1
ion | d. | +1 ion |
|
|
|
6.
|
Of the following, which represents the most stable element? 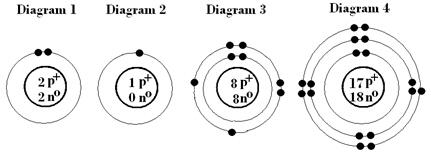 a. | Diagram 1 | b. | Diagram 2 | c. | Diagram
3 | d. | Diagram 4 |
|
|
|
7.
|
Which molecular model is the best representation of NH 3? a. | Model 1 | b. | Model 2 | c. | Model
3 | d. | Model 4 |
|
|
|
8.
|
Which gas could be used in fire extinguishers?
a. | hydrogen | b. | oxygen | c. | methane | d. | carbon dioxide |
|
|
|
9.
|
The diagram shows 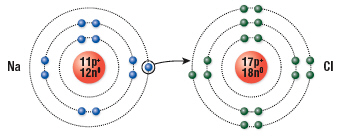 a. | how sodium and chlorine form a covalent bond | b. | how sodium and
chlorine become new elements by exchanging an electron | c. | how sodium and chlorine form an ionic
bond | d. | none of the above |
|
|
|
10.
|
In the diagram, the organism that serves as a food source that would have the
highest concentration of toxins would be at 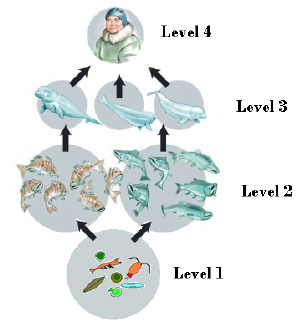 a. | Level 1 | b. | Level 2 | c. | Level
3 | d. | Level 4 |
|